How does soap work?
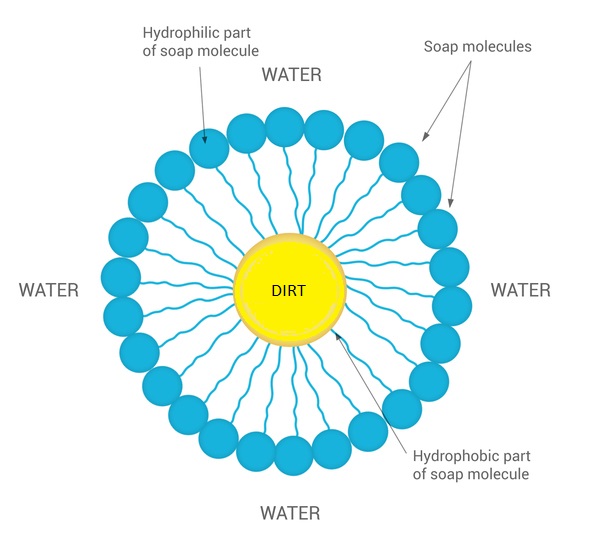
The soap molecule has two reaction capabilities, one is hydrophilic that binds with water and the other is hydrophobic that binds with the dirt particles. The soap molecules creates micelles when they mix with dirt. As the soapy water is rinsed away the dirt goes along with it.This web page represents the agent based simulation model for aforementioned chemical reactions.